Challenge
Avedro: Vedera KXL and Mosaic System
PROJECT
CLIENT
SERVICE
- Platform & Product Development
INDUSTRY
- Life Sciences & Healthcare
Medical startup Avedro sought to commercialize a device that would reshape the cornea with a low-energy microwave innovation.
LASIK vision-correction surgery is an attractive option for many, but the procedure brings with it certain risks. LASIK involves cutting a flap in the cornea and removing a specific amount of tissue to reshape the corneal surface. The incision is then left to heal itself, increasing the risk of infection and side effects such as glare in low-light conditions.
Medical startup Avedro sought to commercialize a device that would reshape the cornea with a low-energy microwave innovation, one whose procedure did not require cutting into the eye, but offered the same level of vision improvement as LASIK. Once a laboratory prototype of this nascent technology demonstrated the desired results, Avedro needed a more reliable instrument for lab researchers to conduct further tests and gather evidence.
Continuum initially created a high-integrity device with flexible parameters and repeatable output with which Avedro extended their research. When the time came to develop a commercial device, Continuum’s team had the requisite knowledge to take on this task, as they understood the foundational strategies required to develop a sustainable solution. The partnership was extended for the next challenge: productizing the technology and bringing it to market.
Research & Insights
Precise Power Management
Precise Power Management
Managing the console’s abundant power source, in relation to the delicate nature of the procedure, was a major technical hurdle. Continuum’s electrical and software engineers created a system that accurately controlled and metered the microwave energy at the tip of the device in real time.
Tools of the Trade
Tools of the Trade
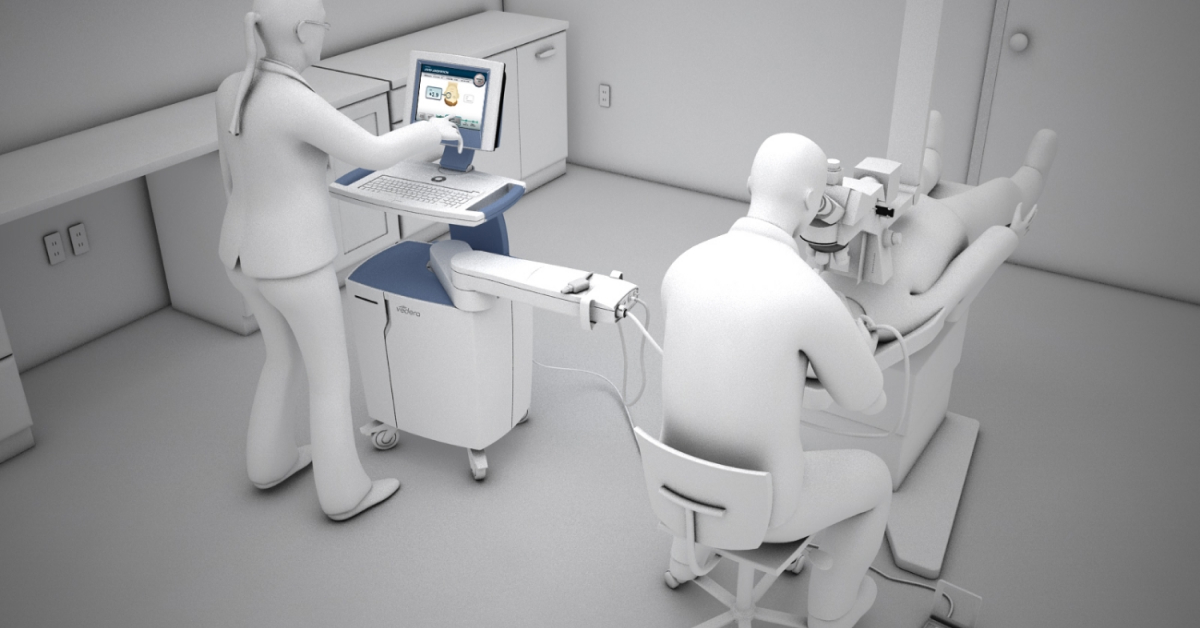
Surgeons needed to be able to lock onto the treatment area of the eye with pinpoint accuracy. Continuum designed early working prototypes for the targeting tools to ensure accurate and precise energy delivery, before going on to develop the remaining patient cabling, support electronics, and fluid systems.
Suited for All Surgical Settings
Suited for All Surgical Settings
From a usability standpoint, the device had to integrate easily within existing surgical suites. Continuum’s team visited refractive surgery centers and interviewed surgeons, nurses, and patients to better understand how each group would interact with the device. The console’s ergonomic design enabled the surgeon to perform the procedure from both the left and right side of the patient, while working in concert with adjacent patient tables and procedure microscopes.
The Human Touch
The Human Touch
During a standard procedure, both the surgeon and a technician operate the console using a touchscreen interface. Continuum applied advanced GUI technologies to create an interface design that was intuitive and easy to use, while also incorporating important safety features that minimized opportunities for errors.
Solution
In one year, Continuum designed and developed a fully functional prototype of the Vedera KXS ready for clinical trials. This revolutionary device reshaped the cornea using microwave technology, so the procedure involved no incision or removal of corneal tissue. The benefits of this treatment over LASIK included equivalent vision improvement with immediate results, faster recovery time, and a much lower incidence of side effects.
While the Vedera KXS proved both safe and effective, continued cell growth in the body meant the results of this new procedure could diminish over time. Avedro had a new technology that saturated the cornea with riboflavin molecules. This process increased collagen cross-linking and strengthened the cornea, thereby extending the longevity of the initial procedure’s effects. Because of Continuum’s familiarity with the initial device and deep understanding of the procedure, the team was able to develop this second “cross linking” instrument with ease.
The KXL and Mosaic Systems are commercially available in the United States as the first FDA-approved therapeutic treatments for progressive keratoconus and corneal ectasia after refractive surgery (LASIK).
Results
After undergoing clinical trials in 2009-2010, the Vedera KXS received the European CE Mark in 2010. The device won a Good Design Award and Medical Design Excellence Award in 2012.
Interestingly, the KXL System and Mosaic System—the two “cross-linking” instruments also designed by the team—have proven to be more marketable solutions for the company. Accelerated cross-linking enabled by the KXL and Mosaic Systems are currently used in multiple countries outside the U.S. to treat kerotoconus, a progressive eye disease for which the only other options are corrective rigid contact lenses or corneal surgery. Accelerated cross-linking has also gained popularity as a follow-up treatment for LASIK, to strengthen the cornea and prolong the treatment’s effects.
The KXL and Mosaic Systems have both received the CE Mark. In June 2015, Avedro announced the sale of their 500th cross-linking device. In 2016, the FDA approved the company's riboflavin ophthalmic solution/KXL System. The KXL and Mosaic systems are now commercially available in the United States as the first FDA-approved therapeutic treatments for progressive keratoconus and corneal ectasia after refractive surgery (LASIK).


